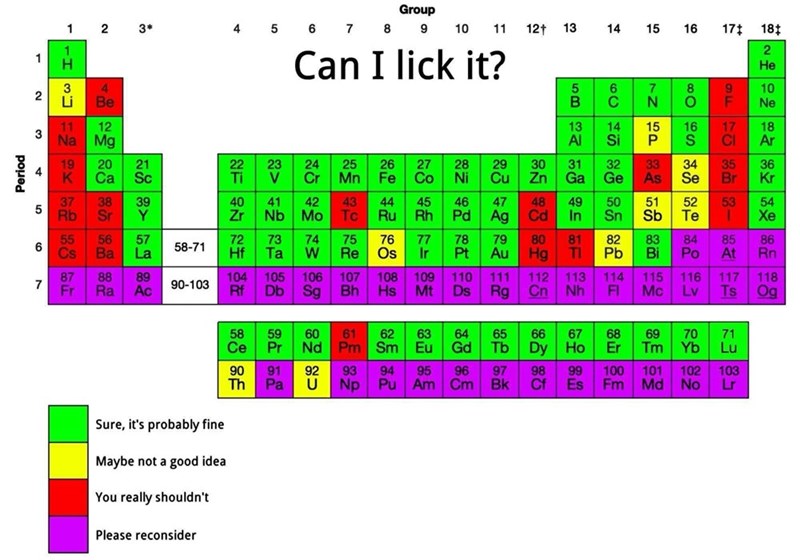
Chemist here: all the reds are correct but it would take so much time to explain why so many of the greens are super concerning. Every time I see this reposted it's so concerning...I should just spend the 17 minutes and save a copy pasta response of everything horribly wrong with this.
Edit: page 1 on the SDS for pure sulfur.